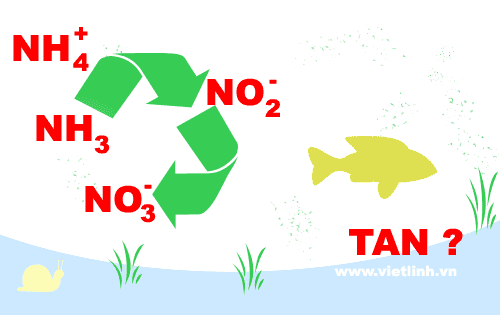
Ammonia (NH3/NH4+):
Ammonia (NH3/NH4+) occurs because of:
• The use of feed for shrimp, fish and aquatic animals
• The decomposition of organic matter in water under the affect of bacteria.
In water, NH3 (gas) exists in equilibrium with NH4+ (ion). NH3 (gas) is toxic to fish and shrimp.
As pH increases, the amount of NH3 (gas) in water increases. If pH is increased by 1 unit, NH3 (gas) will increase 10 times more.
Nitrite (NO2-), nitrate (NO3-):
Ammonia is converted into nitrite (NO2-) due to the effect of nitrosomonas bacteria. Nitrite (NO2-) is then converted into nitrate (NO3-) by the influence of nitrobacter bacteria.
Nitrite (NO2-) is toxic to fish and shrimp because it forms methemoglobin and reduces the transfer of oxygen to animal cells.
To reduce nitrite, chloride can be used.
To limit the amount of ammonia and nitrite (NO2-), nitrate (NO3-), we should:
• Use aquaculture feeds with appropriate protein content.
• Use appropriate amount of feed, avoid excessive feeding.
• Do not use fresh food to feed shrimp or fish.
• Manage algae and pH at stable levels.
• Use paddlewheel aerators or blowers to ensure that dissolved oxygen is above 5 ppm.
• Periodically replace water layer at the bottom of the pond.
© 2016 Viet Linh