H2S (hydrogen sulfide) is a toxic gas, is formed in anaerobic conditions.
In water, H2S (gas) exists in equilibrium with the form HS- ion. If pH is low, the form H2S (gas, toxic) will exist.
For example, at 24°C, if pH = 5, 99.1% hydrogen sulfide is in the form of H2S (gas, poison), the remaining HS-ionic form (non-toxic). If pH = 8, only 8% hydrogen sulfide is in the form of H2S (gas, toxic).
The presence of H2S (gas) in a continuous period will reduce shrimp and fish reproduction although it appears in a very small amount of 0.001 ppm.
H2S concentration in shrimp farming should be less than 0.01 mg/L.
H2S can be eliminated from ponds by aeration or using KMnO4 (potassium permanganate) to oxidize hydrogen sulfide into non-toxic sulfur compounds.
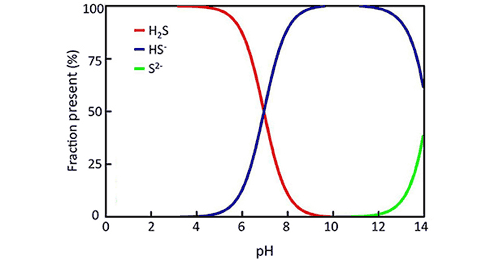
Image: Sulfide intrusion in seagrasses assessed by stable sulfur isotopes - a synthesis of current results
© 2016 Viet Linh